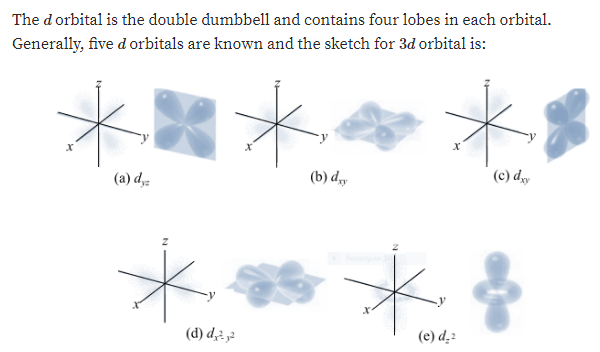
How Would the 4d Orbitals Differ From the 3d Orbitals
Its electronic configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. For example Zinc Zn has atomic number 30.
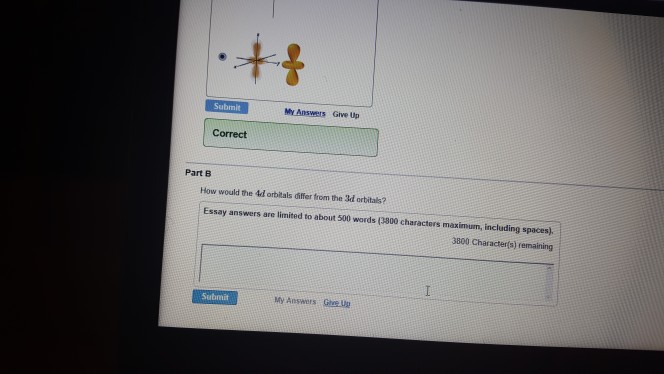
Solved How Would The 4d Orbitals Differ From The 3d Chegg Com
Fill the orbitals according to the atomic number.
. For example the electron configuration of the neon atom is 1s 2 2s 2 2p 6 meaning that the 1s 2s and 2p subshells are occupied by 2 2 and 6 electrons respectively. However their chemical properties differ from the Group 0 elements. In atomic physics and quantum chemistry the electron configuration is the distribution of electrons of an atom or molecule or other physical structure in atomic or molecular orbitals.
Some elements have fully filled valence orbits.

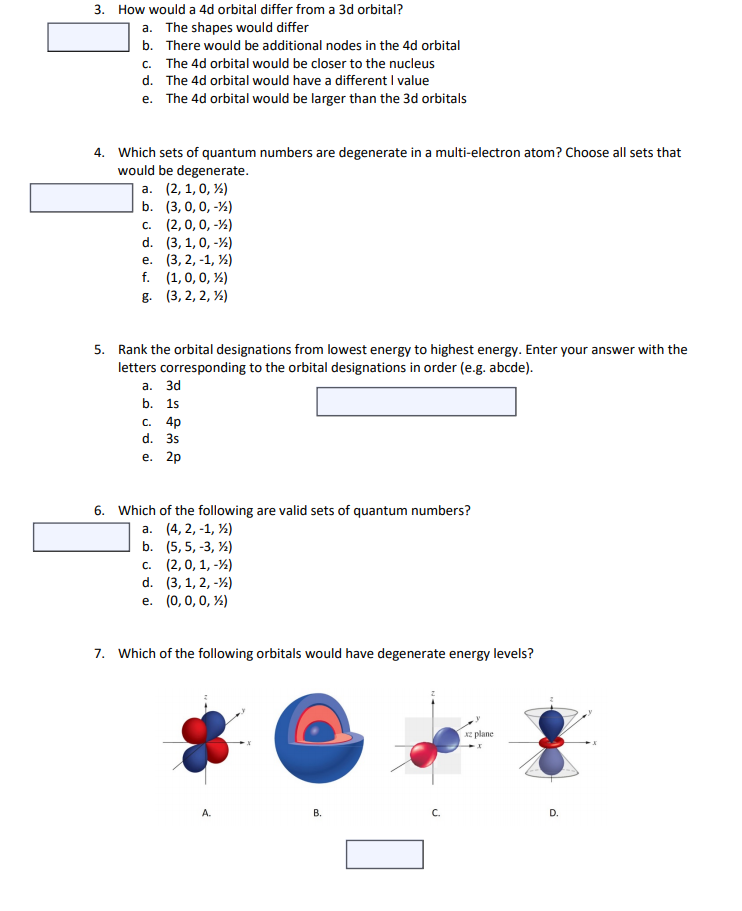
Solved 3 How Would A 4d Orbital Differ From A 3d Orbital Chegg Com
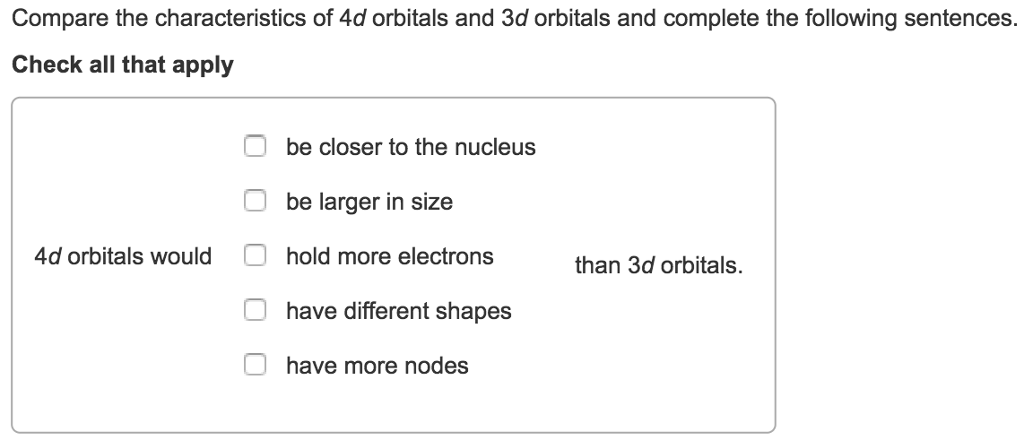
Solved Compare The Characteristics Of 4d Orbitals And 3d Chegg Com
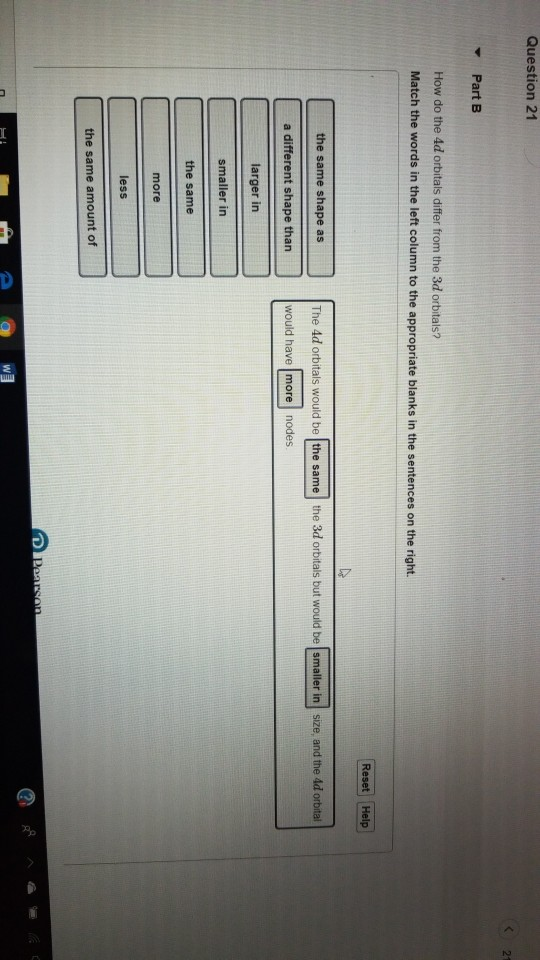
Solved Question 21 Part B How Do The 4d Orbitals Differ From Chegg Com

Comments
Post a Comment